Current battery technology is improving fast, but it still faces challenges like cost, limited materials, and performance in extreme conditions. Relying only on today’s lithium-ion means missing out on solutions that could be cheaper, safer, and more sustainable for many applications.
Exciting new technologies like sodium-ion (including CATL’s Naxtra), molten salt batteries (like NAK chemistry), and others are emerging. They promise lower costs, better safety, wider temperature ranges, and reduced reliance on scarce materials like lithium and cobalt.
I get calls every week from engineers and product developers asking about what comes after lithium-ion. They see the supply chain risks and want to know what alternatives are viable. It is clear that the future isn’t just one type of battery; it’s about having the right tool for the job.
What are the latest market trends for molten salt batteries?
Molten salt batteries, often called high-temperature batteries, are not new, but recent developments are making them more interesting, especially for large-scale storage.
The latest trend for molten salt batteries is a focus on lower-temperature designs and grid-scale energy storage. Companies are developing chemistries, like sodium-metal halide (ZEBRA), that operate at lower temperatures than older sodium-sulfur types, improving safety and cost.

Moving Beyond High Temperatures
Traditional molten salt batteries1 like Sodium-Sulfur (NaS)2 required very high operating temperatures (around 300-350°C). This created safety concerns and required significant energy just to keep the battery hot.
Modern trends focus on:
- Lower Operating Temperatures: Newer chemistries like ZEBRA batteries operate at lower temperatures (around 250°C), making them safer and more efficient.
- Grid Storage Focus: Because they are bulky but can store large amounts of energy cheaply, molten salt batteries are finding a niche in utility-scale projects3 to store solar and wind power.
- Cost Reduction: Research is ongoing to simplify manufacturing and use cheaper materials, making them more competitive against lithium-ion for stationary storage.
Key Players and Applications
| Technology Type | Key Companies | Main Application | Temperature (°C) |
|---|---|---|---|
| Sodium-Sulfur (NaS)2 | NGK Insulators | Grid Storage | 300-350 |
| Sodium-Metal Halide (ZEBRA) | FZSoNick (formerly FIAMM) | Grid Storage, Industrial Backup | ~250 |
While not suitable for portable electronics or most vehicles, molten salt technology is a serious contender for the massive energy storage needed to support renewable energy grids4.
How can you make a salt battery at home?
Making a simple "salt battery" at home is a common science experiment. It shows basic electrochemical principles, but it is very different from commercial battery technology.
You can make a simple salt battery using household items like pennies, zinc washers, cardboard soaked in saltwater, and wires. This creates a very low-power voltaic pile, demonstrating how ions create electricity.

A Simple Science Project (Not a Real Battery)
This experiment demonstrates the concept of an electrochemical cell, but it’s important to understand its limitations.
Materials Needed:
- Copper coins (like US pennies dated before 1982, which are mostly copper)
- Zinc washers (galvanized washers work)
- Cardboard or thick paper towels
- Saltwater solution (dissolve salt in water)
- Wires with alligator clips
- A low-power LED or a multimeter
Steps:
- Cut cardboard/paper towel into small discs, slightly smaller than the coins/washers.
- Soak the discs in the saltwater solution.
- Create a stack: Penny, soaked disc, washer. Repeat this stack multiple times (e.g., 5-10 times). This is your "voltaic pile."
- Use alligator clips to connect a wire to the top penny (positive terminal) and the bottom washer (negative terminal).
- Connect the wires to an LED or multimeter. You should see a small voltage or the LED might dimly light up.
Why This Isn’t Practical Power
This simple setup generates a very small voltage (around 0.5V – 0.8V per penny/washer pair) and almost no current. It cannot power anything significant. Commercial batteries use highly engineered materials and electrolytes to achieve useful power levels, energy density5, and cycle life6. It is a fun demonstration, not a power source.
What should we know about NAK chemistry in batteries?
NAK (Sodium-Potassium alloy) chemistry represents a specific type of molten salt or liquid metal battery technology. It is highly experimental but holds interesting potential.
NAK chemistry typically refers to batteries using a liquid alloy of sodium (Na) and potassium (K) as one of the electrodes. These batteries operate at elevated temperatures and aim for very high power density and long cycle life, often targeted for grid storage.

The Liquid Metal Approach
Using a liquid metal electrode like NAK offers some unique properties:
- High Conductivity: Liquid metals are excellent conductors, allowing for very fast charge and discharge rates (high power).
- Self-Healing: Unlike solid electrodes that can degrade or crack over cycles, liquid electrodes constantly reform, potentially leading to extremely long lifespans.
- Challenges: NAK alloy is highly reactive with air and water, requiring perfectly sealed systems. Like other molten salt types, they typically need to operate at high temperatures.
Research in this area is exploring different designs, including using NAK with molten salt electrolytes or solid electrolytes. It is an advanced field, primarily focused on large-scale stationary storage where the operational complexity can be managed.
How does sodium car battery technology compare with others?
Sodium-ion batteries are starting to appear in electric vehicles, especially in China. How do they stack up against the dominant lithium-ion chemistries?
Sodium car batteries (Na-ion) offer lower cost and better safety than Lithium-ion (like NMC or LFP). Their energy density is currently similar to LFP but lower than high-energy NMC. They perform exceptionally well in cold weather where lithium batteries struggle.

The Sodium vs. Lithium Showdown for EVs
Here is how Sodium-Ion fits into the electric vehicle battery landscape.
| Feature | Sodium-Ion (Na-ion) | Lithium Iron Phosphate (LFP) | Nickel Manganese Cobalt (NMC) |
|---|---|---|---|
| Energy Density | Good (160-175+ Wh/kg) | Good (160-200+ Wh/kg) | Excellent (220-270+ Wh/kg) |
| Cost | Excellent (Lowest) | Good | High |
| Safety | Excellent | Excellent | Good |
| Lifespan | Excellent (5000+ cycles) | Excellent (3000-5000+ cycles) | Fair (1000-2000 cycles) |
| Cold Weather | Excellent (Works at -40°C) | Fair (Reduced capacity) | Poor (Significant reduction) |
| Fast Charging | Excellent | Good | Good |
| Best For | Budget EVs, City Cars, Cold Climates | Standard Range EVs, Commercial Use | Long Range EVs, Performance EVs |
Sodium-ion is not going to replace NMC in high-performance, long-range Teslas tomorrow. But for affordable city cars, commercial vehicles operating in cold regions, or even hybrid packs (like CATL’s AB system), it is a very strong and rapidly improving alternative to LFP.
What are the details and performance of Naxtra sodium-ion batteries?
CATL’s "Naxtra" brand is arguably the most advanced commercial sodium-ion battery currently available. Its specifications set the benchmark for the industry.
CATL’s Naxtra sodium-ion battery boasts an energy density of 175 Wh/kg, an ultra-long cycle life exceeding 10,000 cycles, excellent fast charging (5C peak rate possible), and outstanding low-temperature performance, retaining 90% capacity at -40°C.
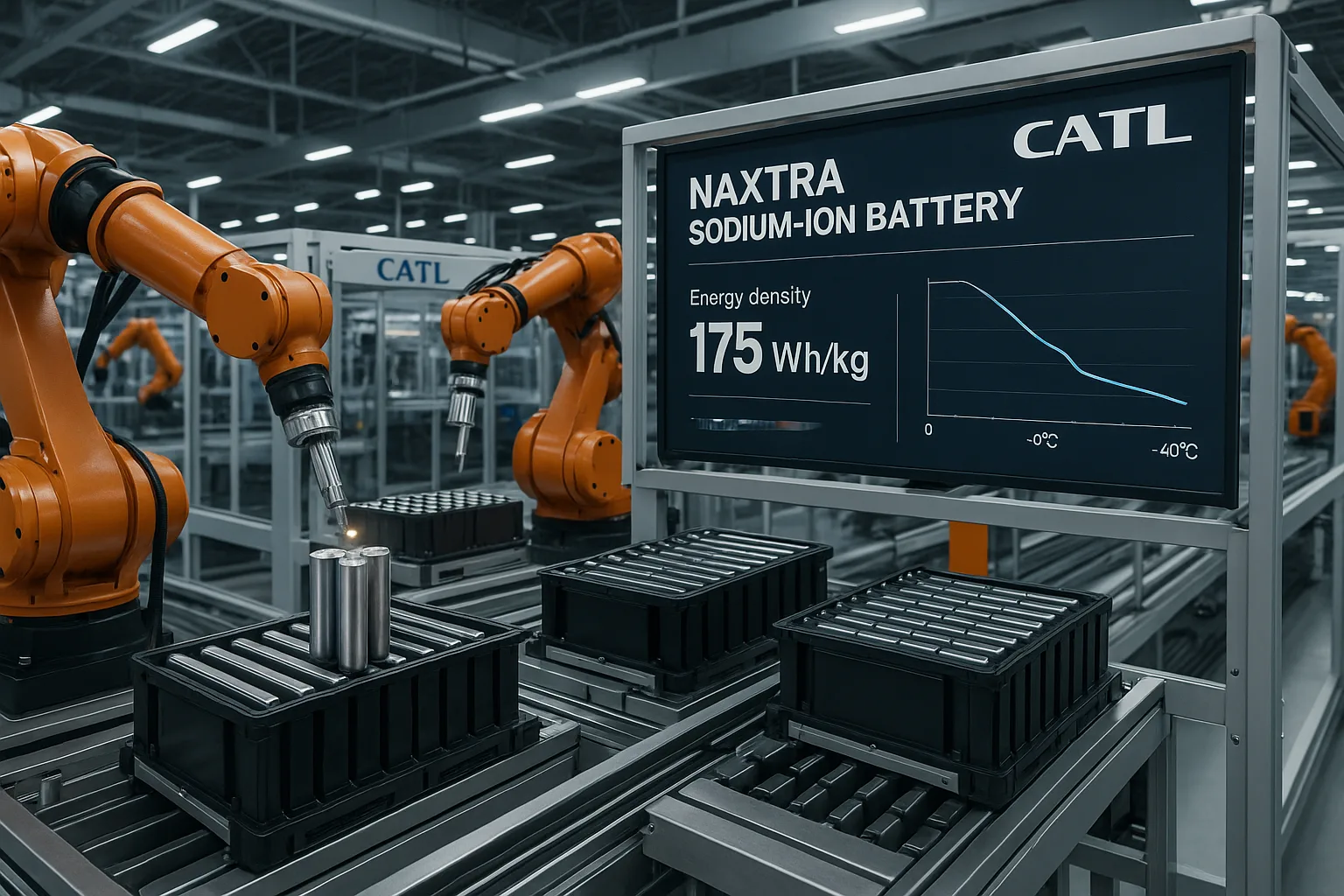
Naxtra’s Key Specifications
CATL has engineered Naxtra to compete directly with LFP on performance while beating it on cost and temperature range.
- Energy Density: 175 Wh/kg (cell level). This allows for EV ranges of up to 500 km (around 300 miles) in optimized packs.
- Cycle Life: >10,000 cycles. This is incredibly high, suggesting a lifespan of decades in many applications.
- Charging: Supports fast charging7, potentially reaching 80% in 15 minutes or less, with peak rates up to 5C.
- Temperature Range: Operates effectively from -40°C to +70°C. This is a massive advantage over lithium-ion, especially for vehicles in cold climates or outdoor energy storage.
- Safety: Meets stringent new safety standards8 (like China’s GB 38031-2025), designed to prevent thermal runaway9.
CATL is mass-producing Naxtra now, initially for specialized vehicles and potentially integrating them into their AB (Sodium + Lithium) hybrid packs for mainstream EVs. Its combination of performance, safety, and low cost makes it a truly disruptive technology.
Conclusion
Emerging battery technologies like sodium-ion and advanced molten salt offer compelling alternatives to lithium-ion. They address key challenges like cost, safety, and material availability, paving the way for more sustainable and versatile energy solutions.
-
Explore the potential of molten salt batteries for large-scale energy storage and their unique advantages. ↩
-
Discover the applications and challenges of Sodium-Sulfur batteries in energy storage. ↩ ↩
-
Explore the role of utility-scale projects in harnessing renewable energy effectively. ↩
-
Discover how renewable energy grids operate and the role of advanced battery technologies. ↩
-
Explore the concept of energy density and its impact on battery performance. ↩
-
Understand the significance of cycle life in determining battery longevity and reliability. ↩
-
Learn about fast charging capabilities and their importance in modern battery applications. ↩
-
Explore the safety standards that ensure the reliability and safety of battery technologies. ↩
-
Learn about thermal runaway and the measures taken to enhance battery safety. ↩