Confused about how sodium-ion batteries are built and function? You’re not alone. Understanding their internal structure and operation can be tricky, especially compared to familiar lithium-ion batteries.
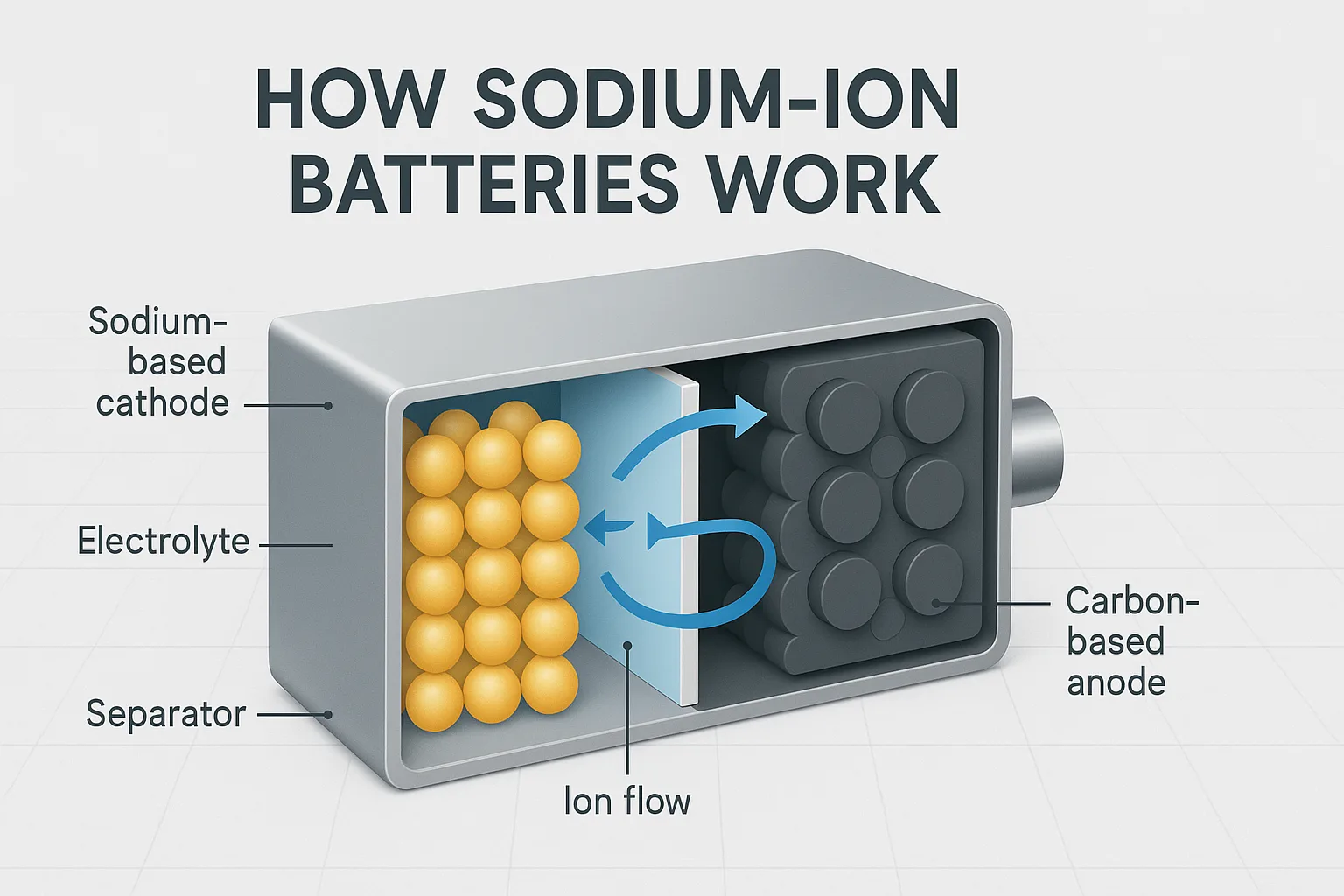
Sodium-ion batteries consist of a sodium-based cathode, carbon-based anode, electrolyte, and separator. They work by transferring sodium ions between electrodes during charging and discharging, similar to lithium-ion batteries.
I recently helped a client grasp battery basics; let’s break down the construction and operation clearly.
What Is the Working Principle of a Sodium-Ion Battery?
Ever wondered precisely how sodium-ion batteries produce energy?
Sodium-ion batteries1 operate through reversible electrochemical reactions2, where sodium ions shuttle between the cathode and anode. During charging, sodium ions move to the anode; during discharge, they return to the cathode, generating electrical energy.
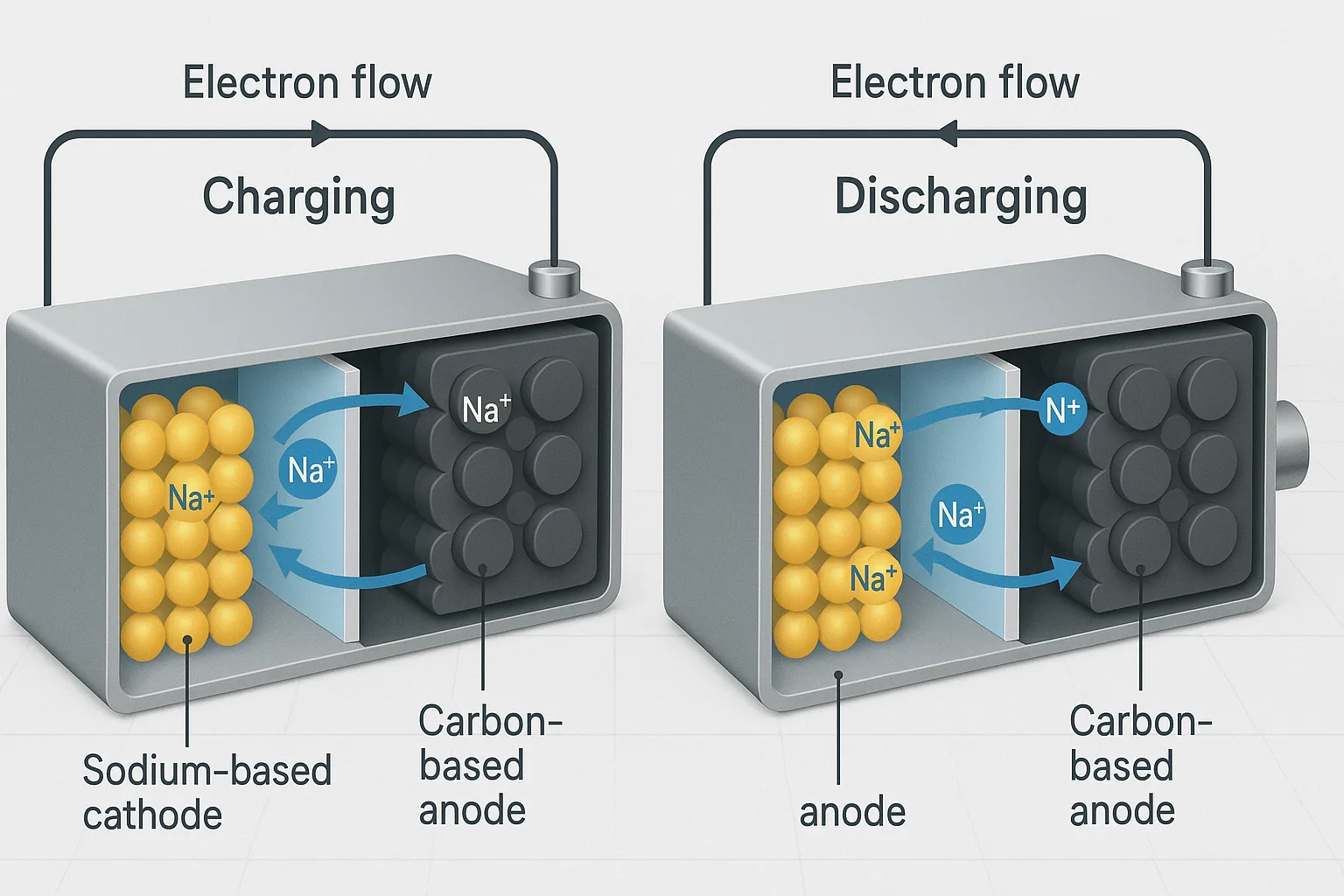
Sodium-Ion Battery Operation Explained
The operation involves these main stages:
- Charging: External energy pushes sodium ions from cathode (positive electrode) to anode (negative electrode), storing energy chemically.
- Discharging: Sodium ions move back from the anode to the cathode, releasing energy to power connected devices.
| Operation Stage | Sodium Ion Movement | Electrode Activity |
|---|---|---|
| Charging | Cathode → Anode (storing energy) | Gains electrons (anode) |
| Discharging | Anode → Cathode (releasing energy) | Releases electrons (anode) |
This shuttling of sodium ions generates usable electricity.
What Chemical Reactions Occur Inside a Sodium-Ion Battery?
Curious about the chemistry behind sodium-ion batteries?
In sodium-ion batteries, sodium ions intercalate (insert) into and deintercalate (remove) from electrode materials. The key reactions involve sodium ions reacting reversibly with cathode and anode materials without permanently changing their structures.
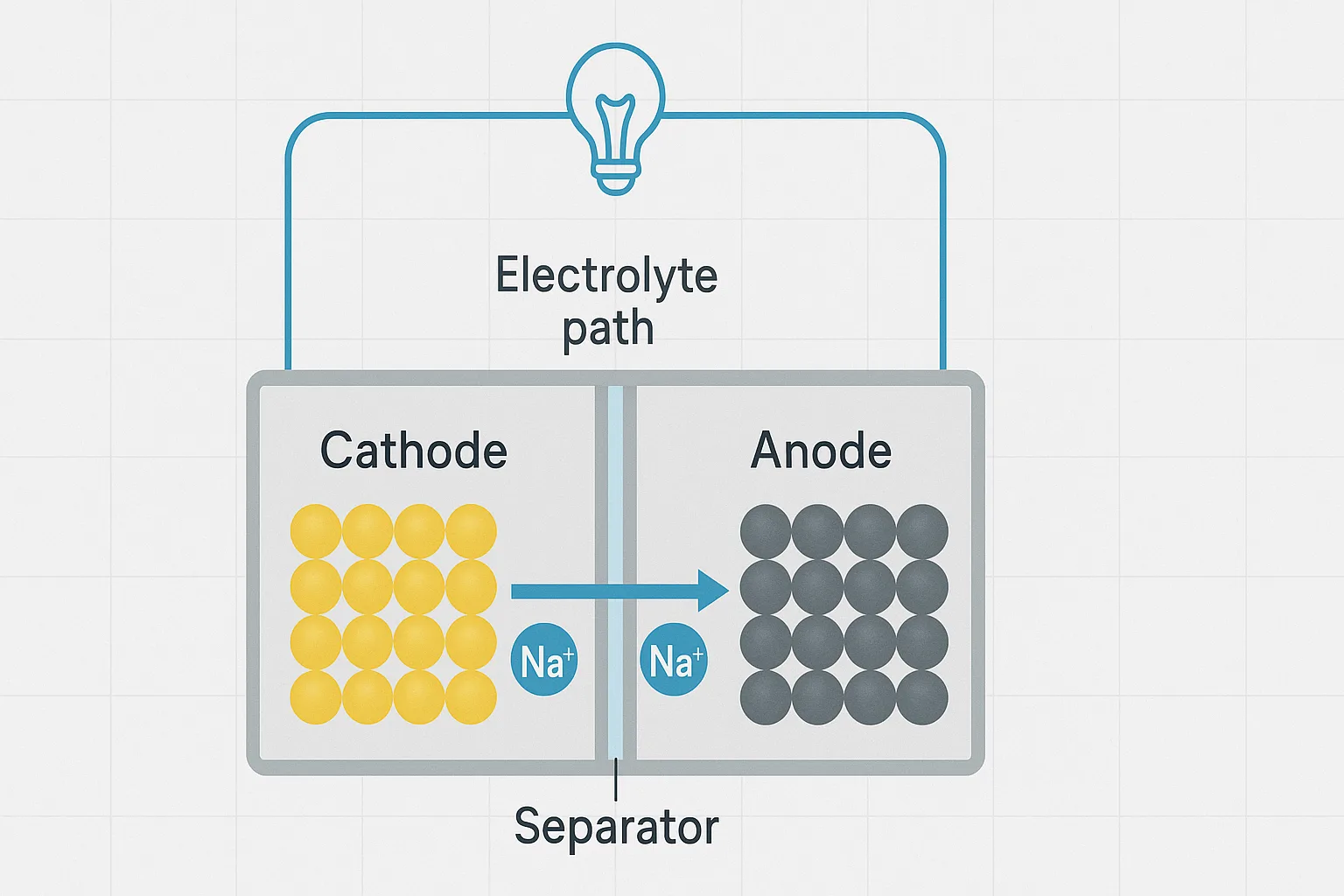
Key Chemical Reactions
Here’s a simplified overview of typical reactions inside a sodium-ion battery:
-
Cathode Reaction:
( \text{Na}_{1-x}\text{MO}_2 + x\text{Na}^+ + xe^- \leftrightarrow \text{NaMO}_2 )
(MO₂ typically represents metal oxide materials.) -
Anode Reaction (Carbon-based):
( \text{C} + x\text{Na}^+ + xe^- \leftrightarrow \text{Na}_x\text{C} )
These reactions are fully reversible, allowing repeated charging and discharging.
What Does a Typical Sodium-Ion Battery Diagram Look Like?
Visualizing the internal components of a sodium-ion battery can simplify understanding its function.
A typical sodium-ion battery diagram3 includes a sodium-based cathode4, carbon-based anode5, electrolyte, separator membrane, and external circuit. Sodium ions flow through the electrolyte and separator between electrodes during charge-discharge cycles.
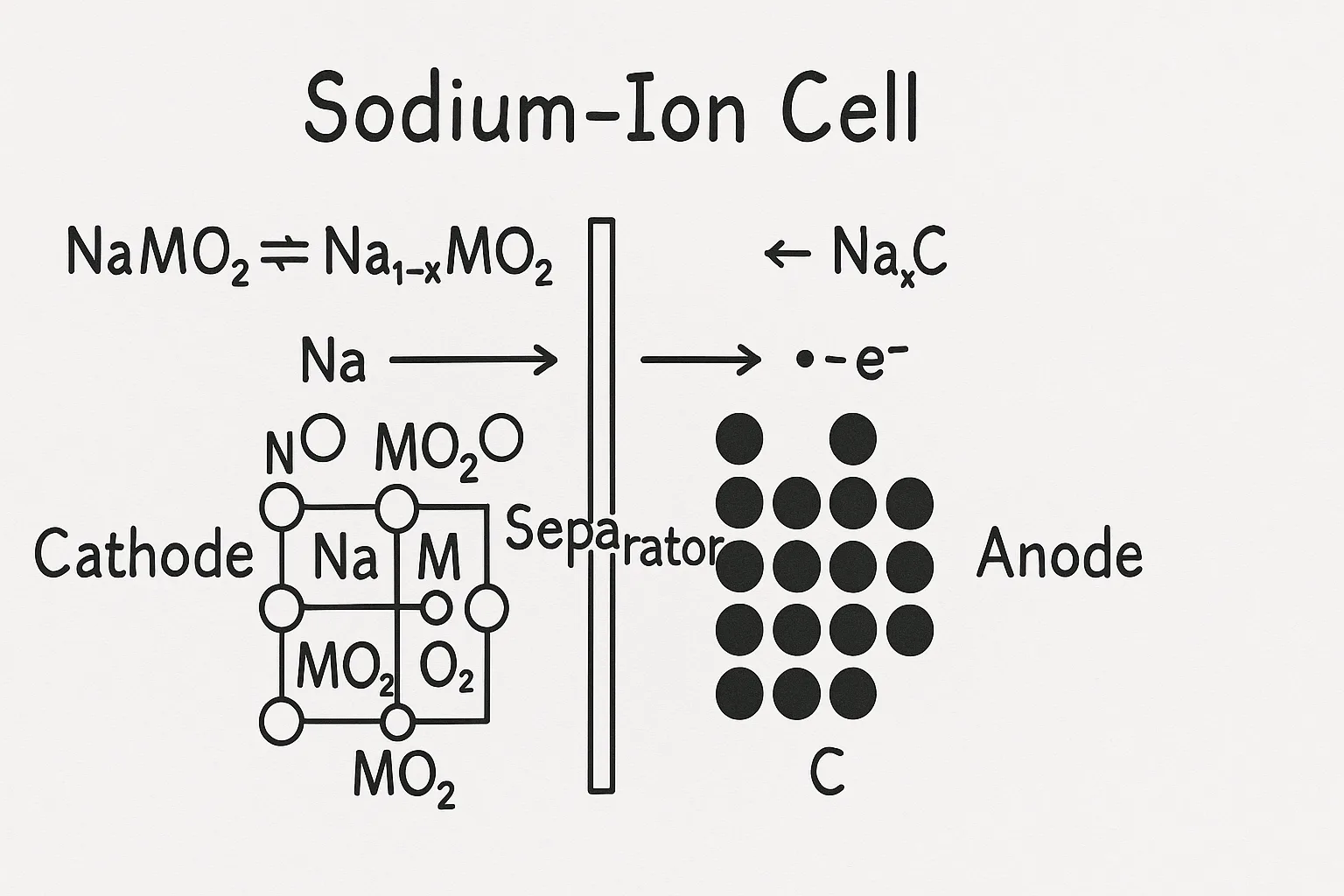
Components of a Sodium-Ion Battery (Diagram)
Here’s a simplified breakdown:
| Component | Function |
|---|---|
| Cathode | Stores and releases sodium ions during cycling |
| Anode | Receives sodium ions during charging |
| Electrolyte | Facilitates sodium ion transfer |
| Separator | Prevents direct electrical contact between electrodes |
| Current Collector | Conducts electricity to external circuits |
Visual diagrams clearly illustrate ion movement and battery operation.
How Does the Internal Structure of a Sodium-Ion Battery Compare to Lithium-Ion?
Wondering how sodium-ion battery structures differ from lithium-ion batteries6?
Structurally, sodium-ion batteries7 closely resemble lithium-ion batteries. Both types have similar components—anode, cathode, electrolyte8, separator—but sodium-ion uses sodium-based materials, resulting in larger battery size and different electrode chemistries.
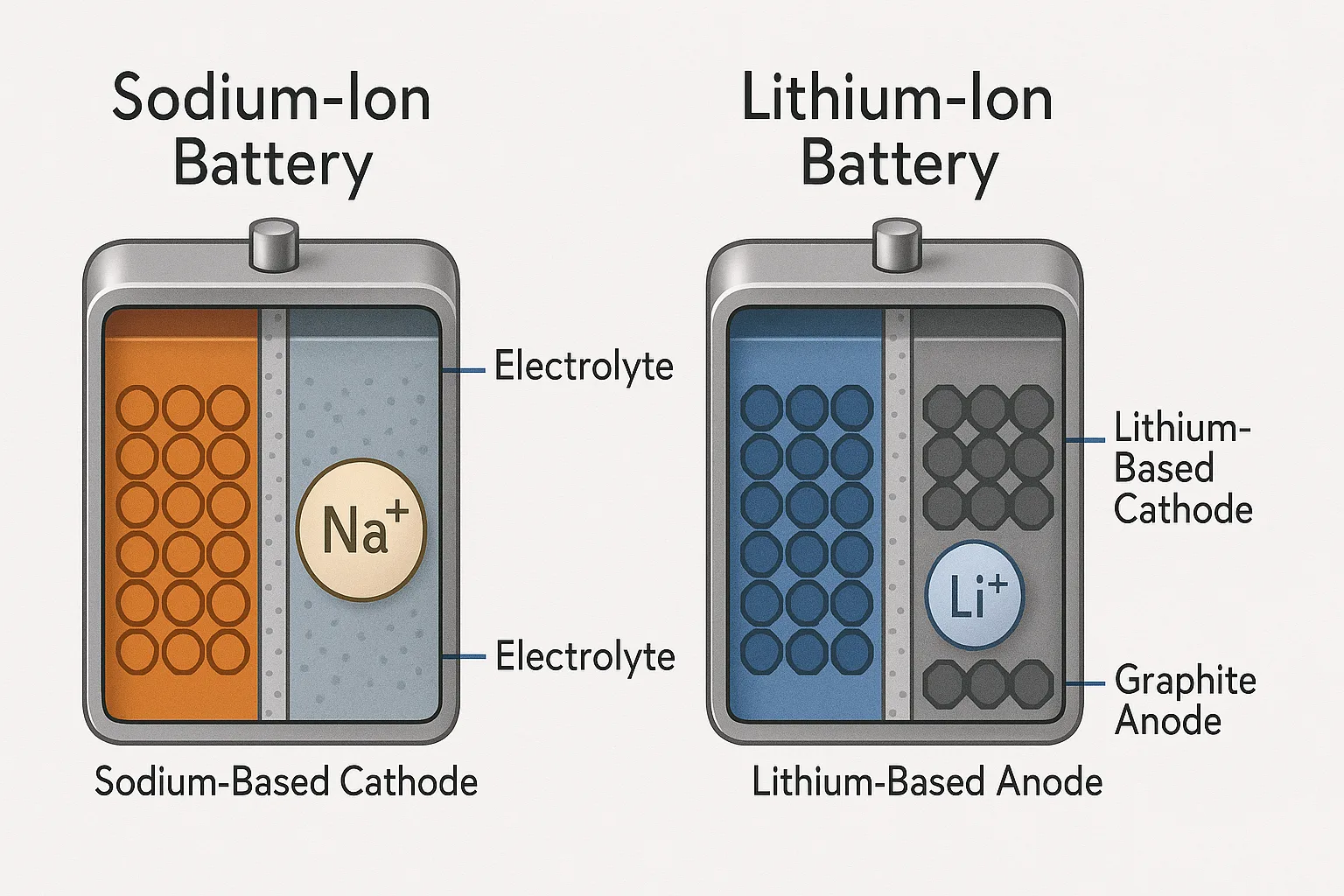
Structural Comparison Between Sodium-Ion and Lithium-Ion Batteries
Here’s a straightforward comparison table:
| Component | Sodium-Ion Batteries | Lithium-Ion Batteries |
|---|---|---|
| Anode Material | Carbon-based (hard carbon) | Graphite or Silicon-based |
| Cathode Material | Sodium-based metal oxides | Lithium-based metal oxides |
| Electrolyte | Sodium salts in organic solvent | Lithium salts in organic solvent |
| Ion size and mobility | Larger sodium ions (slower mobility) | Smaller lithium ions (faster mobility) |
| Energy Density | Lower (due to larger ion size) | Higher |
Despite structural similarities, key differences significantly affect performance and application suitability.
Conclusion
Understanding sodium-ion battery construction, working principles, and chemistry clarifies their potential and limitations. While similar to lithium-ion batteries structurally, sodium-ion’s unique chemistry presents both opportunities and challenges.
-
Explore the benefits of Sodium-ion batteries, including their cost-effectiveness and environmental impact, to understand their potential in energy storage. ↩
-
Learn about reversible electrochemical reactions to grasp the fundamental principles behind battery technology and energy storage solutions. ↩
-
Explore this link to see detailed diagrams and explanations of sodium-ion battery components and their functions. ↩
-
Learn about the role of sodium-based cathodes in battery technology and their advantages over traditional materials. ↩
-
Discover how carbon-based anodes work in sodium-ion batteries and their impact on performance and efficiency. ↩
-
Understanding lithium-ion batteries is essential for grasping their widespread use in technology and energy storage solutions. ↩
-
Explore the benefits of sodium-ion batteries, including cost-effectiveness and sustainability, which are crucial for future energy solutions. ↩
-
Learn about the critical function of electrolytes in battery performance, which is key to understanding energy storage technologies. ↩